Back Titration Method
Titration is a chemical analysis procedure that determines the amount of a samples ingredient by adding a precisely known amount of another substance to the measured sample. The titrant reacts with a solution of analyte.
Difference Between Back Titration And Direct Titration Definition Examples Applications
The chemical of unknown concentration is called the analyte or titrand.
. 50 cm 3 volumetric flask measuring cylinder 5. Small errors in amounts of other substances. Summary of Test Method.
10mL 20mL 25mL pipettes are common. Obviosuly it is important only when transferring sample titrant or stoichiometric reagents used for back titration. The gold standard method to determine the level of somatic activity is by polymerase chain reaction PCR of the target region followed by cloning and sequencing of a large number of clones 2122.
10 cm 3 measuring cylinder. Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a substance to be analyzed. This would be called a split night study.
Nicola is feeling the benefits of quitting smoking after the pandemic made her think more seriously about her health and wellbeing. Background Argentometric Titrations In order for a titrimetric method to be viable the titration reaction 1 must be complete ie Ktitration is. Generates a titration curve from which the endpoint is determined.
The remaining excess reagent is then titrated with another second reagent. Overview of essential cookies. While this is also intrinsic characteristic of the method it can be adjusted for by blind trials.
Then there are errors that can be connected with volumetric glass accuracy. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. The principle is very simple.
Thank you to 2017 Reviewers. In the test an excess of manganeseII salt iodide I and hydroxide OH ions are added to a water sample causing. This method becomes necessary if.
Titration is also known as titrimetry or volumetric analysis. The volume of titrant that is reacted usually to produce a color change is called the titration volume. The more I realised it was the right quitting method for me.
Thank you to 2020 Reviewers. E203 Test Method for Water Using Volumetric Karl Fischer Titration. Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration Version.
The sample is weighed into a headspace vial and closed with a septum cap. Thank you to 2019 Reviewers. Thank you to 2021 Reviewers.
For the potentiometric method an automatic titrator will be used to perform the titration and to obtain the titration curve. Thank you to 2018 Reviewers. In this method an excess of a standard solution of EDTA is added to the metal solution being determined so as to complex all the metal ions present in the solution.
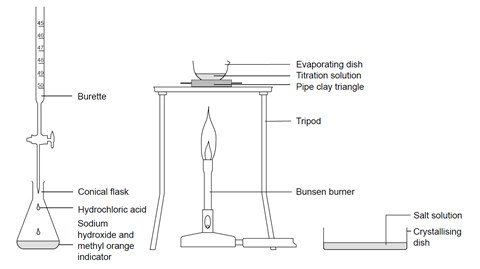
A brief note about analytical cookies. Back to McClements Home Page Back to FD SCI 581 Page. We use a pipette to measure out a known volume of the sample solutionThis is a much more accurate measurement than if we used a measuring cylinderA pipette is a glass tube with a fixed volume pipettes are available in a range of volumes eg.
The Winkler test is used to determine the concentration of dissolved oxygen in water samples. That is a user needs to find the concentration of a reactant of a given unknown concentration by reacting it with an excess volume of another reactant of a. When sleep apnea is diagnosed during the overnight sleep study a titration is then performed to determine the optimal CPAP pressure setting required to resolve apnea episodes.
The Lane-Eynon method is an example of a tritration method of determining the concentration of reducing sugars in a sample. When placed in an oven the water evaporates and a carrier gas usually air or nitrogen dried with a molecular sieve transports the released water into the titration cell where the determination of the water content takes place. This chemical called the titrant or titrating solution reacts with.
We also need some glassware and apparatus to carry out the titration. The water is separated. Sometimes the titration is performed during the second half of the overnight sleep study.
Xultophy 10036 is not recommended as first-line therapy for patients who have inadequate glycemic control on diet. To figure out the amount of titrand in the solution a known amount of a different chemical is added to the titrands solution. An ample supply of QC sample material shall be available for the intended period of use and shall be homogeneous and stable under the anticipated storage.
Thank you to 2016 Reviewers. A reagent termed the titrant or titrator is prepared as a standard solution of known concentration and volume. Although this method has high sensitivity and specificity to determine the activity level it is both expensive and labor-intensive.
Acidified ethanedioic acid solution CORROSIVE causes severe skin burns and eye damage Note 1manganeseII. Your doctor may prefer a 2 night sleep study with the first. The procedure is.
Everything You Need to Know About Peer Review The Good The Bad and The Ugly. Titration is a method commonly used in chemistry to figure out the amount of a chemical in a solution. The gravimetric method or more sophisticated chemical methods eg the Theander-Marlett method.
Investigate and take corrective action to bring the test back into control before proceeding. Save Exit. Dissolved oxygen DO is widely used in water quality studies and routine operation of water reclamation facilities to analyze its level of oxygen saturation.
About Open Access. Back Cookies used on this site. Burette 50 cm 3.
The excess of EDTA left after the complex formation with the metal is back titrated with a standard solution of a second metal ion. Xultophy 10036 insulin degludec and liraglutide injection 100 unitsmL and 36 mgmL is a combination of insulin degludec and liraglutide and is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. This chemical is called the titrand.
Or if access becomes slow or incomplete due to system back-up procedures Internet traffic volume upgrades overload of requests to servers general network failures or delays or any other cause that may from time to time make the. Reviewer Resource Centre. Test tubes with rubber bungs.
What is Back Titration It is basically an analytical technique in chemistry which is performed backwards in the method. Potassium manganateVII solution 0002 mol dm-3 no hazard. A standard solution of a reagent of known concentration is called the titrant or titrator.
ARM Project Home Info for Volunteers Results pH and Alkalinity Lake Sampling Method - River Sampling Method - Analysis Protocols - pH Electrode Maintenance For the Mass. Department of Environmental Protection-approved SOP get the pdf file for lakes or for rivers Background Information pH is a measure of the hydrogen ion concentration of the water as ranked on a.

Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education

What Is The Use Of Titration How Do You Explain Back Titration Quora


Comments
Post a Comment